Why a back pressure regulator is useful in continuous flow control
The effectiveness of a continuous flow process is determined by the precision and consistency of the flow measurement and control equipment. Therefore, it is critical to choose the appropriate control valve for the application. Many flow control schemes use variable orifice control valves in conjunction with flow transmitters and a closed-loop PID controller. An alternative method of flow control is to employ a dome-loaded back pressure regulator (BPR) to control flow by controlling the pressure drop across an orifice. By using a BPR, the flow control will be buffered from downstream changes in flow and pressure, creating a more consistent continuous flow process.
Equilibar has unique dome-loaded, multiple-orifice control valve technology that offers superior precision and frictionless control, especially for low flow rates, mixed phase fluids, corrosive media, and extreme temperatures.
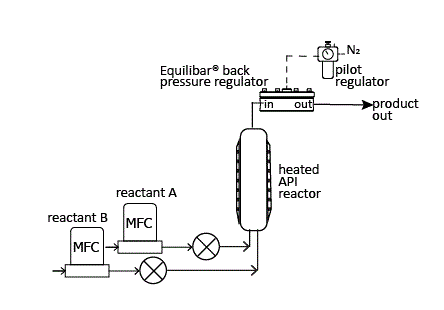
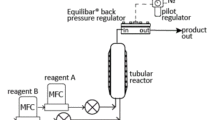
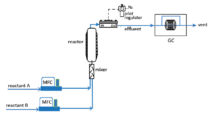
In the schematic above, two reactants are pumped continuously into a heated API reactor. Here the Equilibar® back pressure regulator provides a stable flow through the reactor that ensures a steady residence time and reaction speed, resulting in the desired full reactant conversion and consistent final product.
The ability of the Equilibar BPR to control the process at high temperature and high pressure increases effective residence time and reaction rate. Precise pressure control keeps the reaction at the desired phase throughout the process. Together these qualities deliver higher product yield and consistency, resulting in lower cost and better quality.
Certification Considerations in API Manufacturing
API manufacturers must adhere to strict safety and quality standards set by the country where it will be sold. Manufacture of products used in the US will be overseen by the FDA. If the API will be used in Europe, manufacturers must meet regulations of the European Medicines Agency.
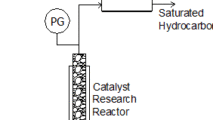
Many API’s are manufactured using catalyzed reactors that require stable pressure control at elevated temperatures. Some formulations require highly corrosion resistant metals such as Hastelloy C276 and elastomers such as Kalrez FFKM. Equilibar’s simple design consists of one moving diaphragm and an optional wetted O-ring. This allows Equilibar to adapt standard designs to meet demanding requirements of pharma. Equilibar diaphragms can be made of FDA and European Medicines Agency approved materials.
Certifications for Equilibar back pressure regulators are available upon request to conform to the requirements certifying agencies.
Contact an application engineer for more information about using an Equilibar back pressure regulator in your continuous flow chemistry or API process.
Learn more about our back pressure regulator technology and how it works.
Watch this video how the Equilibar Back Pressure Regulator works.