Single Use Valve
The Equilibar® dome loaded multi-orifice valve technology is now available in a single use regulator. The patent pending Equilibar SDO Series valves are manufactured using USP Class VI polymers and stainless steel. The disposable polymer body is inserted into a stainless steel support housing with wing nut closure for quick and easy assembly. The Equilibar SDO is a revolutionary design for single use bioprocess control and can be used in pressure or flow control applications in place of a standard pinch valve where accurate results are required.
Contact Us to learn more about SDO
Equilibar SDO Valves 1″, 3/4″, 1/2″, 3/8″, 1/4″ and 1/8″ hose barb fittings
Alternative to a Single Use Pinch Valve
Single-Use Pinch valves use external mechanical compression over existing product tubing to control flow and pressure through a single-use process line. The Equilibar single use valve is a very precise alternative to a pinch valve. Its award-winning design allows it to be configured for precision pressure regulation or closed loop flow control. Compared to a pinch valve, the SDO single use back pressure regulator responds to process fluctuations and setpoint changes more quickly.
Applications
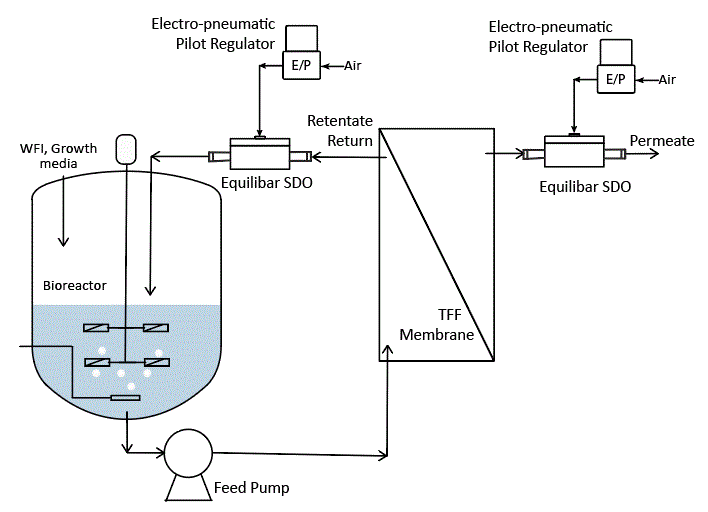
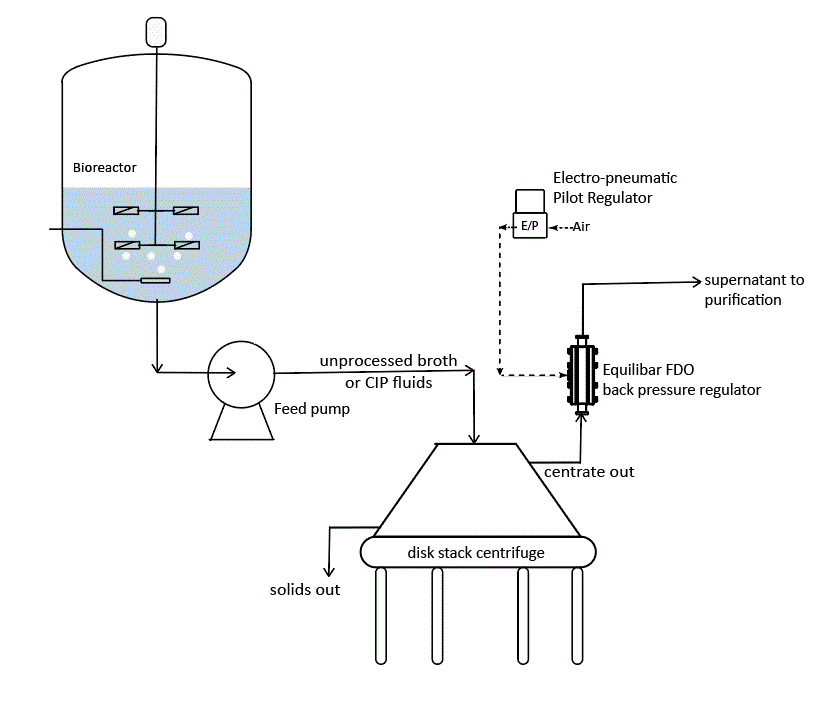
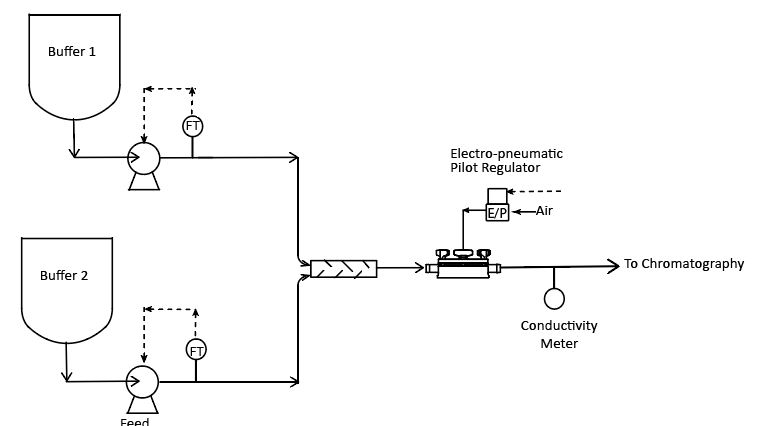
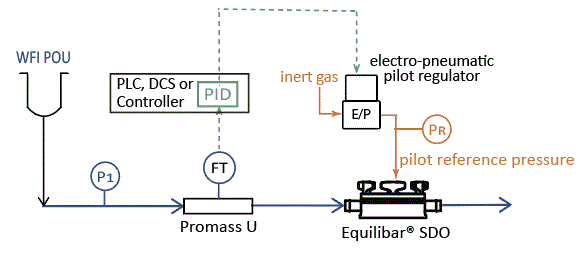
The Equilibar SDO delivers process advantages in many single use applications where pressure and flow are critical factors such as tangential flow filtration, disc stack centrifuge, buffer delivery in chromatography, and WFI point of use flow control.
SD Series Specifications
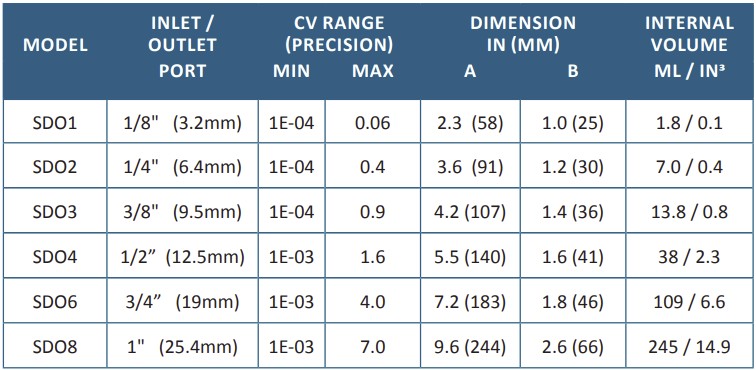
DIM A is takeout length from inlet tube fitting to outlet tube fitting. DIM B is the height, not including wingnuts. To download .stp files, use link button to specific SDO size product page.
How it Works
The SD single use regulator features Equilibar’s patented dome-loaded multiple orifice technology delivering instantaneous control, frictionless operation and superior precision across exceptionally wide flow rate ranges. The stainless steel housing is designed as a permanent part of the process and connects to a dome pilot pressure supply. The disposable body is sold in a pre-sterilized state and comes ready for sterilization up to 50 kGy. See Sample Certificate of Conformance.
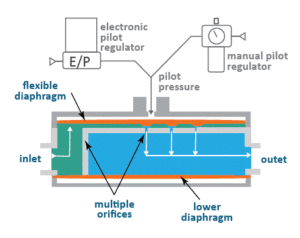
Cutview schematic of Equilbar SDO with dome loaded multi-orifice design
Once assembled, simply apply a pressure to the pilot port in the dome of the Equilibar SDO valve equal to the desired setpoint and the valve does the rest. This pilot pressure forces the upper flexible diaphragm down onto a field of orifices. A rise in inlet pressure lifts the diaphragm up to allow excess pressure to be relieved through the outlet orifices. Similarly, a loss of pressure at the inlet causes the diaphragm to be pushed closer to the orifices, restricting flow and rebuilding pressure at the inlet.
SDO 3 Valve with Polymer Replacement
Looking for Multi-Use Sanitary Valves?
SDO3 – 3/8” hose barb fitting
View Product
SDO4 – 1/2” hose barb fitting
View Product
SDO6 – 3/4” hose barb fitting
SDO8 – 1” hose barb fitting
View Product
Equilibar SDO Offers Easy Maintenance, Precision and Stability
Video demonstrating the ease of operation, instantaneous response and precise pressure control.
Equilibar SDO Valves for Single Use Flow Control
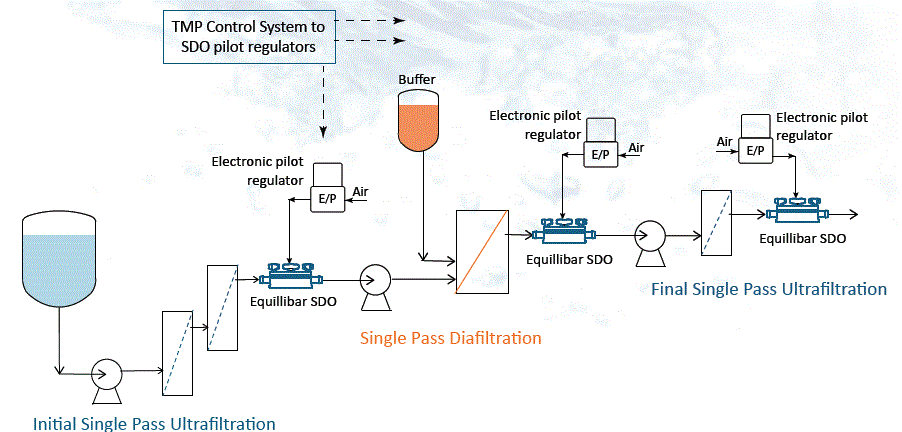
Using the same SDO valve cradle and consumable insert, the Equilibar SDO valve can be used as a flow control valve for single use bioprocessing systems. Configure the SDO valve in a closed loop configuration with an electronic pilot pressure regulator (E/P) and a flow meter in a PID feedback control loop.
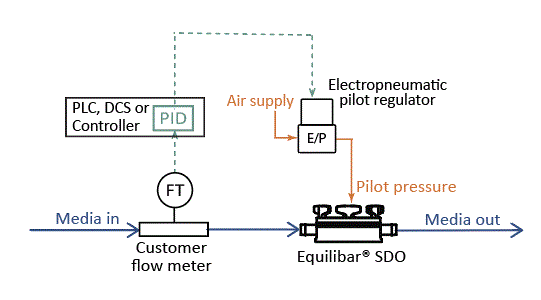
Equilibar SDO in Single Use Flow Control Configuration
A proportional-integral-derivative (PID) controller monitors the input from the flow transmitter (FT) and sends a signal to the electronic pilot regulator (EPR). The EPR translates the electronic signal from the PID into a pressure signal that adjusts the pilot pressure to control flow through the SDO.
This unique configuration offers precision control with 100:1 flow turndown that can’t be matched by automated pinch valves, or automated weir style single use valves. The SDO valve can control flow across a wider range of flow rates than other single use fluid control device and it has extremely fast response.
Where Equilibar Single Use Flow Control Valves Make a Difference
Buffer delivery systems in gradient elution applications can benefit from using the Equilibar SD valve to control flow for precision blending. The SDO can also offer many advantages in Water-for-injection (WFI) points of use flow control.
Learn More About Using Equilibar Valves for Flow Control
Single Use On/Off Valve
The Equilibar SD can be used for on/off situations in single use processes. SD advantages for on/off applications include its simple construction, ease of computer automation and low pressure drop.
Looking for a pulsation dampener for single use bioprocessing?
Equilibar engineers have leveraged their fluid control knowledge to design an active pulsation dampener for single use bioprocessing systems. This single use pulsation dampener uses pneumatics and self-adjusts its setpoint to match system pressure in real time to dampen pulsations. This behavior is similar to how the Equilibar SDO back pressure valve dampens pulsations, but is optimized for this dedicated dampening device.
The non-wetted sides of the diaphragms are pressurized with air through a pressure regulator connected to a port on the top and bottom of the stainless steel housing. This patented air balancing system adjusts its internal pressure in response to the force and direction of each pump pulsation against the diaphragms, reducing pulsation amplitude. Early trials have shown >70% reduction on amplitude, and establishes a more uniform pressure profile.