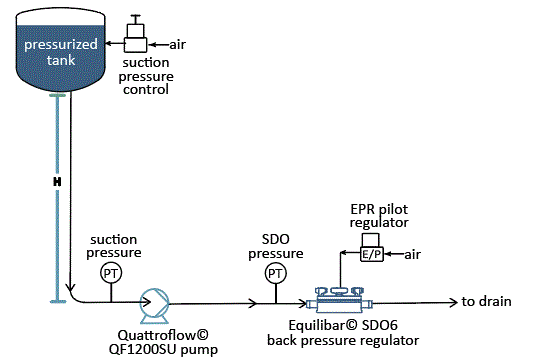
A collaborative paper by Ryan Heffner, Equilibar’s Single Use Technology Manager, and researchers with PSG Biotech reports on a promising method of preventing push-through flow in diaphragm pumps, particularly when feed tanks are at levels higher than the process area, as is often necessary due to clean room space limitations. While this study focuses on single-use systems, the methods will also work in multi-use systems.
(Go directly to paper here.)
Background:
Diaphragm pumps are popularly used to provide reliable and efficient transfer of aqueous solutions and biologics in single-use and multi-use biopharma manufacturing, especially for chromatography, tangential flow filtration, and centrifuge feed applications.
In applications where the feed tank is below or level with the diaphragm pump, the pump creates a suction lift to prime the fluid into the pump so that the diaphragm action feeds the fluid gracefully from lower pressure at the inlet to higher pressure at the outlet.
Applications where feed fluids are above the pump are more complex. This process configuration is not uncommon since limited clean room space requires feed fluids to be stored at elevated heights to save floor space for other production needs. Buffers, for example, are often stored on a level above the pump that sends fluids to a chromatography or filtration skid. The geodetic height of these buffers above the pump’s suction port creates hydraulic pressure–often referred to as elevation pressure or Net Positive Suction Head (NPSH)–which in turn pushes the check valves open, causing fluid to flow through the diaphragm pump chamber. In this scenario, uncontrolled or unwanted flow passes through the diaphragm pump so that the pump is unable to control flow below the push-through rate, thereby decreasing the practical control range of the pump.
Push-through flow can lead to product waste or inaccurate buffer dilution and decreased yield.
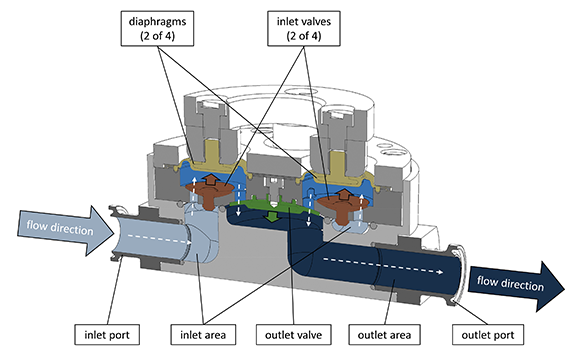
Sectional drawing of a QF1200SU pump chamber illustrating a flow path through a diaphragm pump with diaphragms and inlet check valves. When high inlet pressure occurs, fluid pushes the inlet check valves open. Photo credit: PSG Biotech
Solution:
Engineers at Equilibar and PSG Biotech explored a single-use specific method for preventing problematic push-through flow in applications involving tanks stored at levels higher than the process area. In their tests, they placed a sensitive dome-loaded multiple orifice back pressure regulator (BPR) at the outlet of the pump to prevent unwanted flow. Adding this type of BPR downstream of the pump increased outlet pressure above the elevation pressure so that the full control window of the pump could be realized independent of NPSH.
In this study, an Equilibar® SDO6 single-use BPR was placed downstream of a PSG Biotech Quattroflow® single-use QF1200SU diaphragm pump. Results showed that the setup enabled the pump to maintain zero flow when necessary. In addition, the low flow rate range is more accurate so that the full range of flow rate can be used. The paper includes specific experiment data as well as detailed explanations showing the advantages of the specific pump and BPR used in testing. These methods can be used in single use or multi-use systems.
Read the paper in full — Equilibar Valves Prevent Pump Push-Through Flow
For more information on Equilibar solutions to complex fluid control solutions
Ryan has years of experience in finding elegant solutions for the most complex fluid control challenges in pharmaceutical manufacturing. He will be available to discuss this study and other fluid control issues at the following shows:
- ISPE CaSA Tech Show, Raleigh, NC, February 25, 2025 Table 833
- BPI West in San Diego, March 19-21, 2025 Booth 334
- BPI East in Boston, September 15-18, 2025 Booth 723
Ryan and all our application engineers invite you to contact them about your complex fluid control challenges. They look forward to hearing from you!
Contact Us